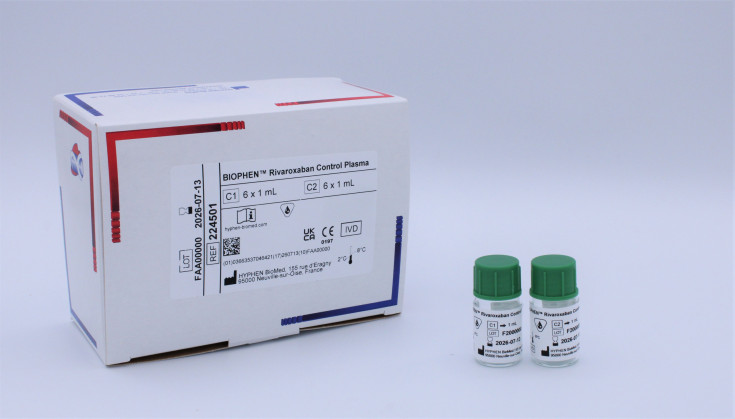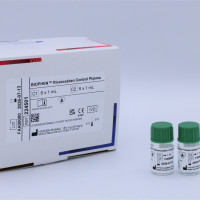
BIOPHEN™ Rivaroxaban Control Plasma
BIOPHEN™ Rivaroxaban Control Plasma
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
A lyophilized human plasma control set for the quality control of Rivaroxaban assays. Intended for use with anti-Xa chromogenic methods on quantitative automated systems to validate assay compliance and homogeneity.
Product Overview:
BIOPHEN™ Rivaroxaban Control Plasma is a set of lyophilized human plasma controls intended for the quality control of Rivaroxaban assays using a quantitative automated method. These controls are proposed for the quality control of anti-Xa chromogenic assays of Rivaroxaban in plasma (BIOPHEN™ DiXaI (221030) and BIOPHEN™ Heparin LRT (221011/221013/221015)). In vitro diagnostic kit for professional use only.
Key Features and Benefits:
- Lyophilized Human Plasma: Ensures stability and mimics patient samples for accurate quality control.
- Two Concentration Levels: Provides controls at two distinct concentrations (approx. 100 ng/mL and 300 ng/mL) to check assay linearity and accuracy.
- Traceable to Standards: Controls are traceable to the European Pharmacopoeia (Ph. Eur.) Certified Reference Standard for Rivaroxaban.
- Broad Assay Compatibility: Validated for use with key anti-Xa chromogenic assays, including BIOPHEN™ DiXaI (221030) and BIOPHEN™ Heparin LRT (221011/221013/221015).
Principle and Application:
These controls are used for the quality control of anti-Xa chromogenic assays for Rivaroxaban in human citrated plasma. While Rivaroxaban monitoring is not typically required, quantitative measurement may be of use in certain clinical situations, such as in the event of emergency surgery or suspected overdosage (bleeding risk).
Product Composition and Specifications:
This kit contains lyophilized human plasma controls with stabilizing agents.
- C1: Lyophilized human plasma containing approximately 100 ng/mL of Rivaroxaban.
- C2: Lyophilized human plasma containing approximately 300 ng/mL of Rivaroxaban.
Note: The BIOPHEN™ Rivaroxaban Control Low (225101) is also available.
Traceability and Performance Characteristics:
Controls are traceable to the European Pharmacopoeia (Ph. Eur.) Certified Reference Standard for Rivaroxaban. Certificate of traceability and uncertainty is available on the manufacturer’s website.
- Lot-to-Lot Variability: %CV ≤ 10% (measured on 3 lots).
- Uncertainty:
- C1: ± 15 ng/mL
- C2: ± 15 ng/mL
Storage and Stability:
- Unopened Vials: Store at 2-8°C. Stable until the expiration date printed on the label.
- Reconstituted Vials: Stable for 7 days at 2-8°C, or 60 days frozen at -20°C or less (thaw only once).
Disclaimer:
The information on this page is for informational purposes and serves as a general summary. It is imperative that all users read and strictly follow the Instructions for Use (IFU) document provided with the reagent or kit they are using. This online description must not be a substitute for the official product documentation.
-
Product RangeProduct Code224501Product NameBIOPHEN™ Rivaroxaban Control PlasmaProduct CategoryProduct BrandProduct Analyte or ApplicationProduct IVD Registration StatusCE-Mark - Professional Use
UKCAProduct Size2 x 6 x 1 ml -